
Congenital LungDiseases
Adam Guttentag M.D.




Congenital Lung Diseases
•Developmental anomalies
–Aplasia/hypoplasia/horseshoe lung
–Foregut malformations
•TE fistula
•Sequestration spectrum
–Vascular anomalies (veins, arteries,both)
•Surfactant deficiency
–Prematurity
–Abnormal surfactant production
•Thoracic anomalies—secondary lunghypoplasia
–Congenital diaphragmatic hernia
–Skeletal dysplasias

Developmental Anomalies:The “Sequestration Spectrum”
•Bronchopulmonary Sequestration
•Congenital Cystic AdenomatoidMalformation
•Congenital Lobar Emphysema
•Bronchogenic Cyst
•Scimitar Syndrome
•Pulmonary AVM

Congenital Lung Anomalies may include:
•Dysplastic pulmonary tissue
•Anomalous arterial supply
•Anomalous venous drainage
•Communication with bronchial tree or GI tract
•Gross structural lung anomalies
•Diaphragmatic defects
•Categorization depends on combination of findings,but may be difficult (“overlap syndromes”)

“Sequestration Spectrum”
pulmonary
vascular
Bronchogenic cyst
CCAM
CLE
Sequestration
Scimitar syndrome
AVM’s

Embryology
•Foregut
–Esophagus, tracheobronchial tree, stomach, duodenum
•GI tract and tracheobronchial tree separate at 26 days
•Primitive lung buds send branches into lung mesoderm up to16th week
•Peripheral bronchi form acinar structures 16th-24th weeks
•Saccular proliferation and alveolar development up untilbirth
•Connections of mesodermal vasculature to primitive aortanormally regress
•Failure to regress may lead to abnormal systemicconnections

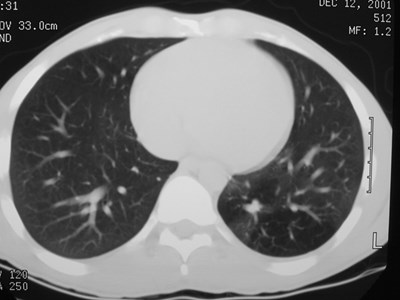
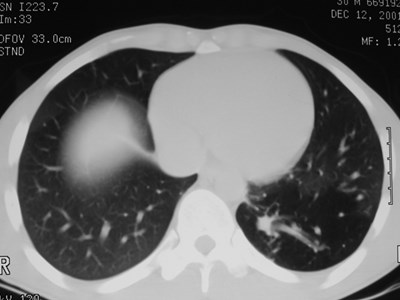
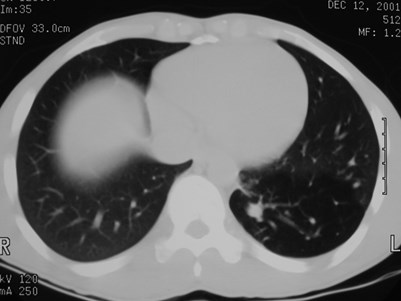
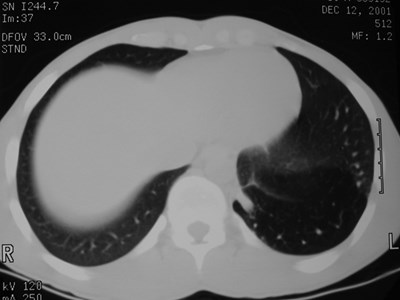

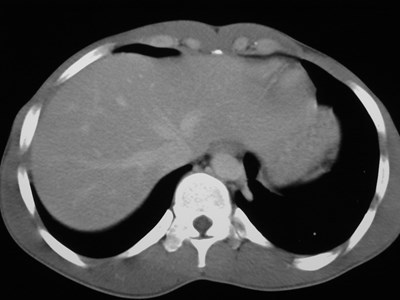
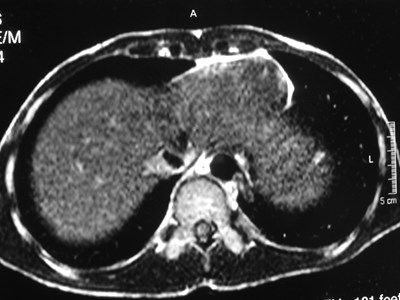
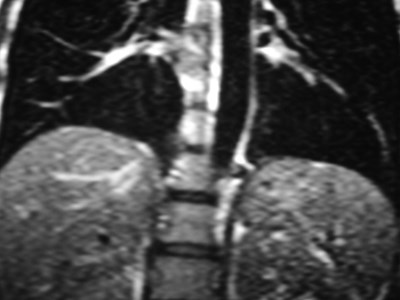

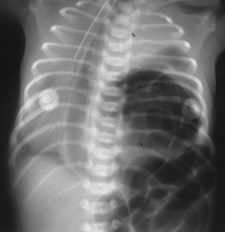
Sequestration
•Abnormal lung tissue without normalbronchial connection and with systemicarterial supply
•Intralobar 75%
•Extralobar 25%

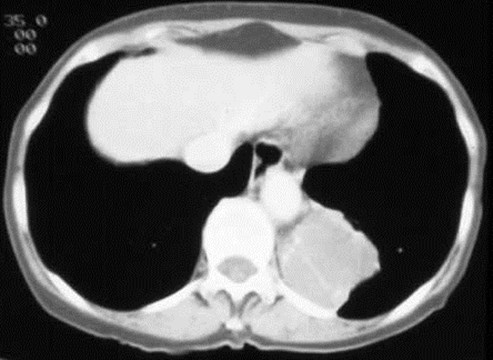
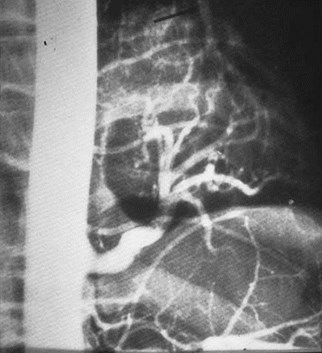
Sequestration - ImagingFeatures
•Abnormal opacity in medial lung base on CXR
•CT shows abnormal lung parenchyma well
•Arteriography classically used to show systemicsupply, venous drainage
•MRI or CT now used to evaluate for systemic arteryand should replace arteriography
•US prenatal / neonatal --homogeneous echogenicity(ELS)

Intralobar Sequestration
•Probably acquired in most cases
–Very rare in infants
–Only 50% discovered before age 20
•Sx: cough, sputum, recurrent pneumonia
•Etiology - many hypotheses
–early bronchial obstruction with recurrent infection
–chronic inflammation
–parasitization of systemic vessels in the pulmonaryligament

Intralobar Sequestration
•Above and contiguous with the diaphragmwithin otherwise normal lung
•Systemic arterial supply
–73% descending aorta
–21% upper abd aorta, celiac, or splenic artery
–16% multiple supplying arteries
•95% with pulmonary venous drainage(L to L shunt)
•98% in lower lobes (60% on L)

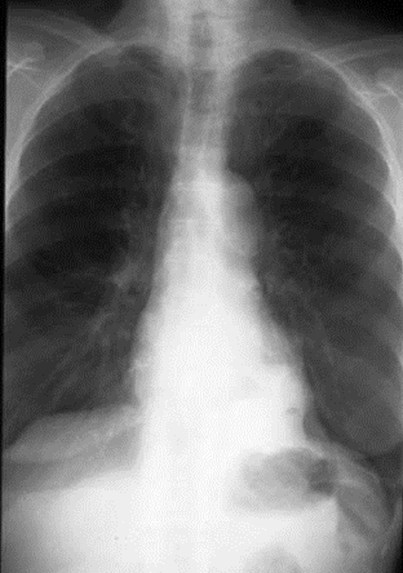
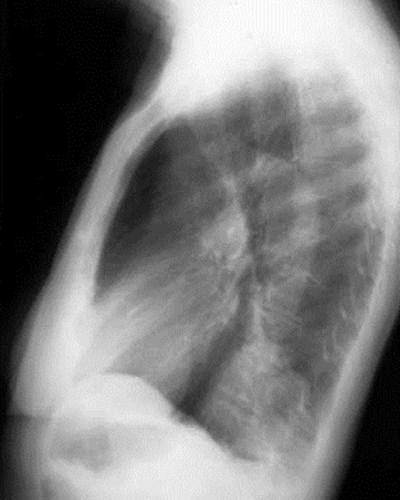
Intralobar SequestrationRadiology
•Usually appears as space occupying massor persistent consolidation
–Purely soft tissue vs. air containing
–Often multiple air-containing cystic areas

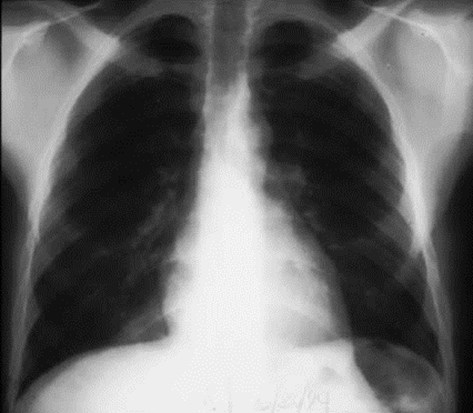
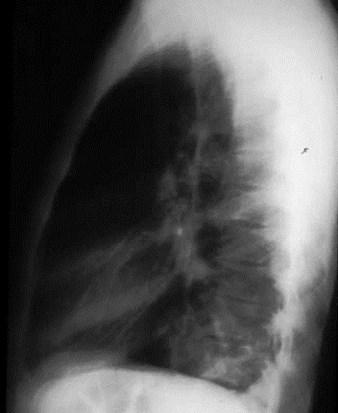
6/74

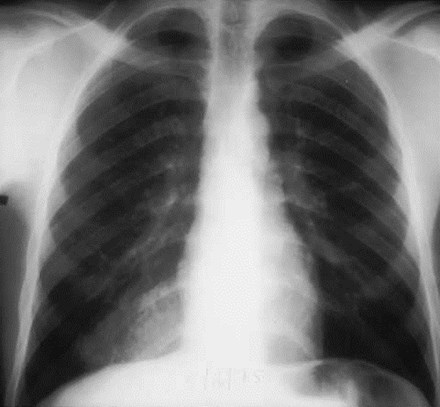
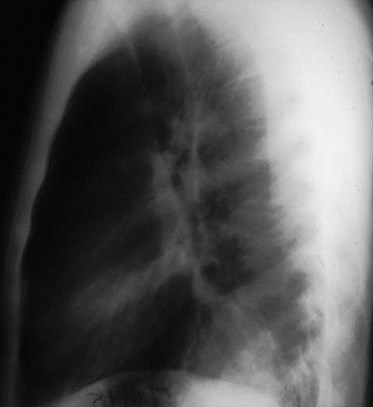
5/75

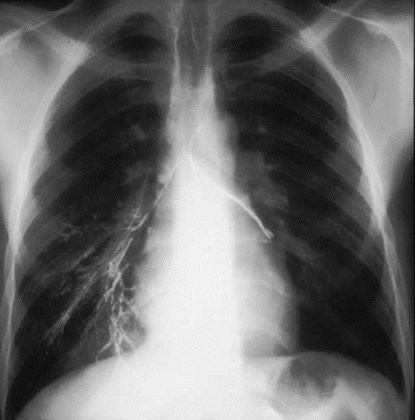
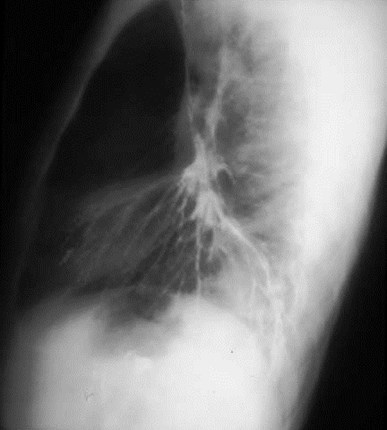

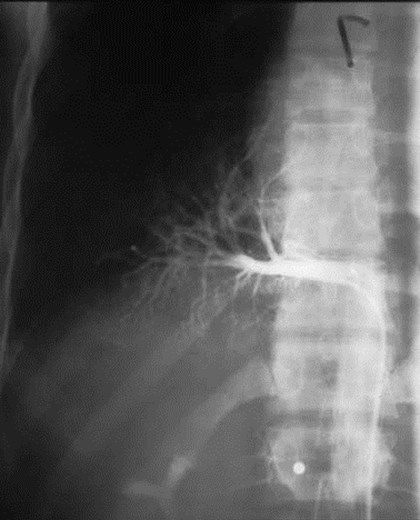

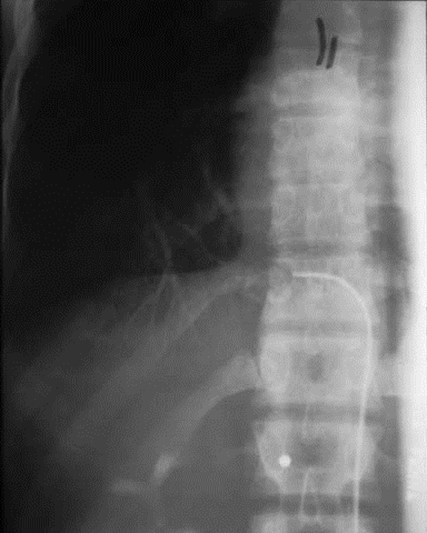
Pulmonaryvenous drainage

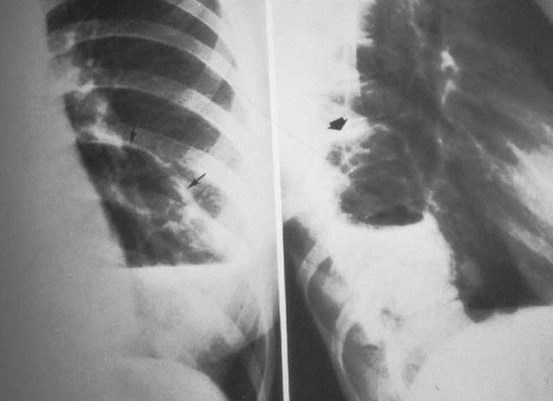
Intralobar sequestrationwith bronchialcommunication

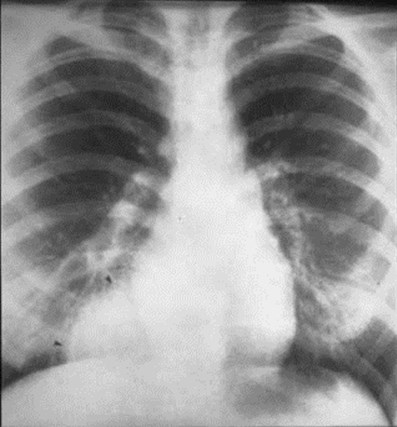



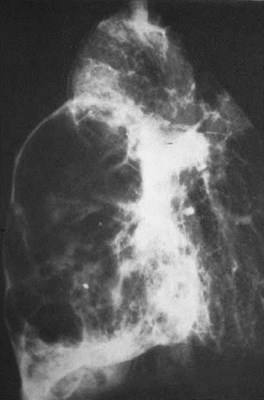
Extralobar Sequestration
•6% of congenital lung lesions
•65% with associated congenital anomalies (CDHmost common)
• M:F = 4:1
•Etiology: probably abnormal budding fromprimitive foregut forming accessory lung with itsown blood supply and pleural covering
•Communication with GI tract rarely persists

Extralobar Sequestration
•Separate visceral pleural covering
•90% on left, almost always in medial basebetween lung and diaphragm
•95% with systemic arterial supply
•>80% with systemic venous drainage (azygos)
•Unusual locations
–Mediastinum, retroperitoneum, pericardial space,diaphragm
•May have assoc. bronchogenic cyst, CCAM

Extralobar Sequestration -Clinical
•Sx: dyspnea, cyanosis, feeding problems
•61% discovered by age 6 mo.
•Only 10% Asx (much less than ILS)
•Rarely presents as recurrent infection

Extralobar Sequestration -Radiology
•May be seen in utero as a mass or inassoc. with polyhydramnios or hydrops
•High output CHF
•Solid mass in LLL


SequestrationIntralobar vs Extralobar
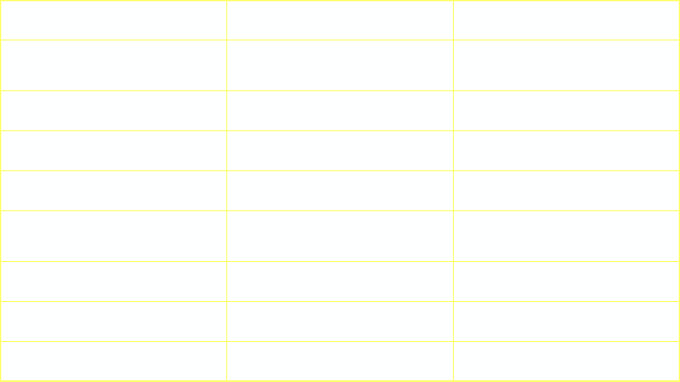
Feature
Intralobar
Extralobar
Pleura
Within normal lobarpleura
Separate pleuralinvestment
Sex incidence
M=F
M>F
Laterality
60% on left
80-90% on left
Arterial supply
Systemic
Systemic
Venous drainage
Pulmonary vein
Azygos, hemiazygos orportal vein
Foregut communication
Very rare
More common
Other congenital anomalies
Uncommon
Common
Diagnosis in neonates
Rare
Common

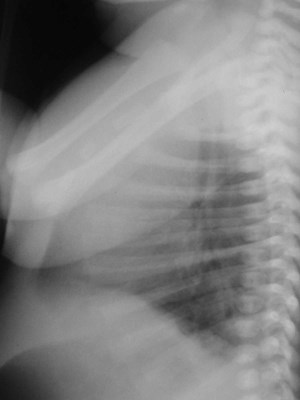
Neonate with abnormal prenatal US

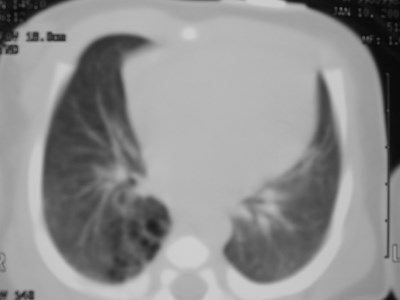
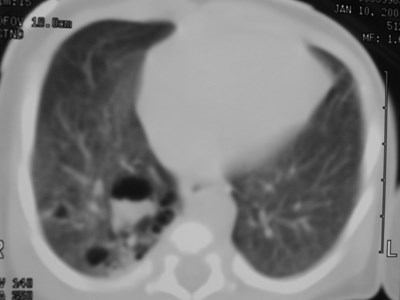
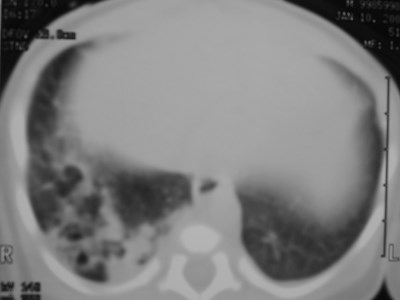

Congenital CysticAdenomatoid Malformation(CCAM)
•Congenital dysplastic lesion of lung
•25% of congenital lung lesions
•Communicate with bronchial tree
•Pulmonary arterial and venousconnections
•R=L Upper lobes predominate slightly

CCAM - Clinical Features
•M=F
•Majority present in neonatal period
•Acute respiratory distress
•Decreased breath sounds, cardiacshift, hyperresonance
•Large lesions may cause hypoplasiaof normal lung
•Unusual cause of fetal hydrops

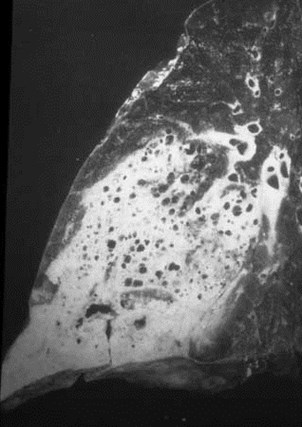
CCAM - Subtypes
•Type I: one or more large cysts (>2cm) withrespiratory epithelium. >50% of cases.
•Type II: cysts all <2cm, cuboidal epithelium.Associated with other severe congenitalmalformations. 40% of cases
•Type III: tiny cysts, usually <5mm. Usuallyinvolves entire lobe or lung. <10% of cases.

CCAM - Prenatal Imaging
•Polyhydramnios, fetal hydropssecondary to obstructed venous return
•Mass may be echogenic (type III) orshow cysts of varying size and number(types I and II)
•10 - 20% shrink on follow-up
•Prenatal DDX: bronchogenic cyst,sequestration, CDH

CCAM - Post-natal Imaging
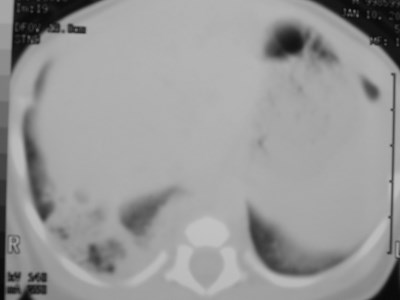
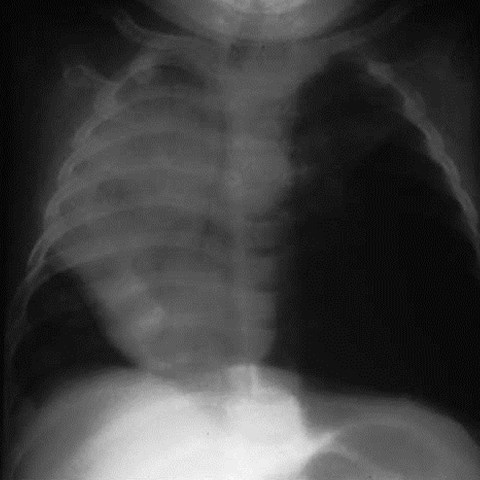
Type I: unilateral air-filled lesion. May be very largeand herniate across the midline.
–Cysts may progressively expand and worsen respiratorydistress.
–Initially may appear solid due to retained fluid in cyst(s).
–Air fluid levels may be seen.
•Type II: Small uniform cysts may be seen.
•Type III: Solid mass or consolidation is usualappearance. Generally large.

CCAM - Treatment andPrognosis
•Treatment: surgical removal--risk of infectionor neoplasm
•Fetal surgery or shunting may be considered toprotect unaffected lung for large masses.
•Prognosis: depends on size of lesion and statusof unaffected lung.
•Poor prognosis if fetal hydrops present.
•Type II lesions may have worse prognosis dueto assoc. renal and cardiac anomalies.

Air filled type I CCAM

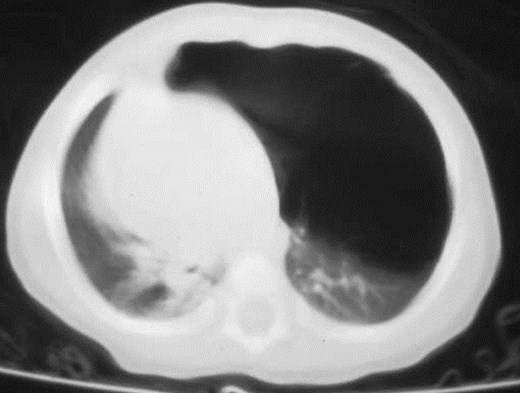
Air filled Type I CCAM

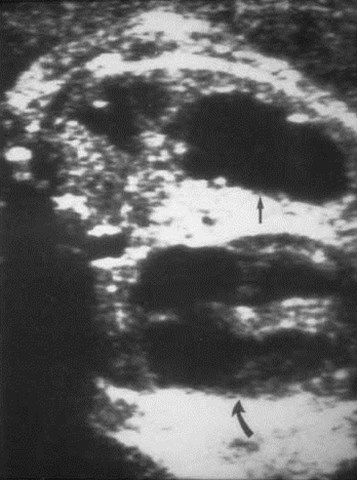
Prenatal US type ICCAM
heart

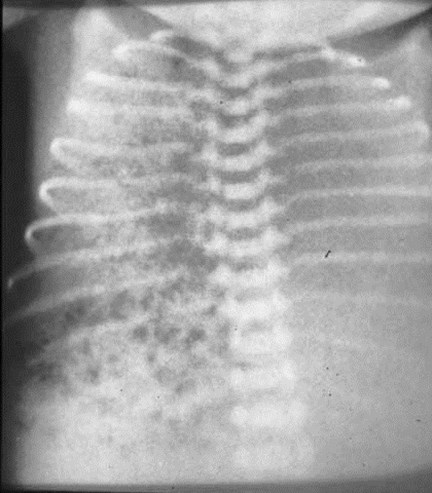
Bubbly type II CCAM
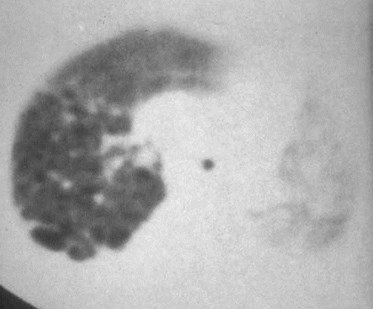

CCAM - Differential Dx
•if air filled CLE
•if solid by US Sequestration(extralobar)
•if bubbly CDH
•if single large cyst Bronchogenic cyst
•All can present in neonate as a mass onCXR
•CT is often helpful in making the dx



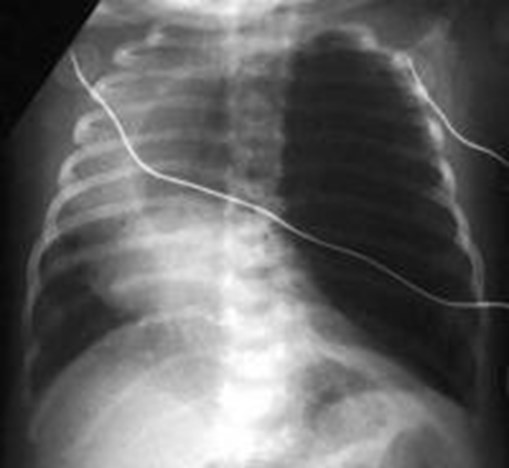

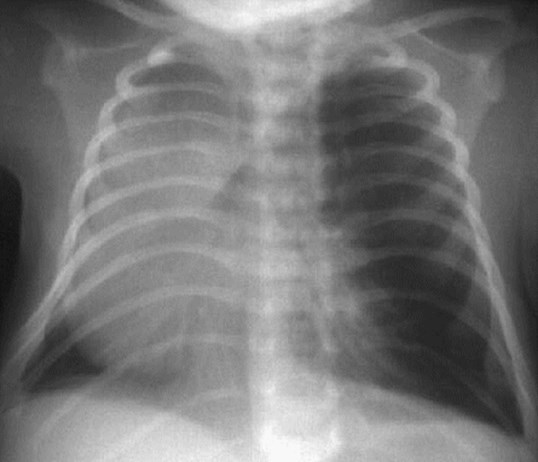
Congenital Lobar Emphysema
•a.k.a. ”neonatal lobar hyperinflation”
•Overinflated lobe with compression of normallung
•Causes:
–Abnormal bronchial cartilage with atresia orobstruction
–50% with bronchial obstruction
–Abnormality of lung development e.g. too many ortoo few alveoli
•LUL 43% RML 35% RUL 20%

Congenital Lobar Emphysema
•Usually presents within 1-2 months asrespiratory distress.
•In bronchial atresia/compression,aeration is via collateral air drift.
•Associated cardiac anomalies
•May do well with conservativemanagement.

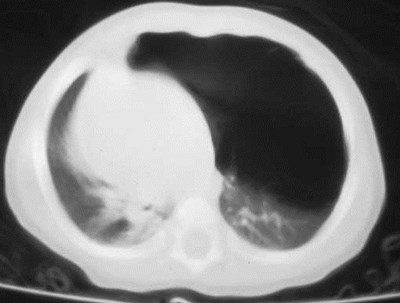
Congenital Lobar EmphysemaRadiology
•Hyperlucent and expanded area of lung actingas a mass
•Maintains size on expiratory or decubitus view
•“Mediastinal swing” on fluoroscopy
•CXR may show a “solid” mass initially
•CT shows normal vessels in hyperexpandedlobe

CLE of LUL

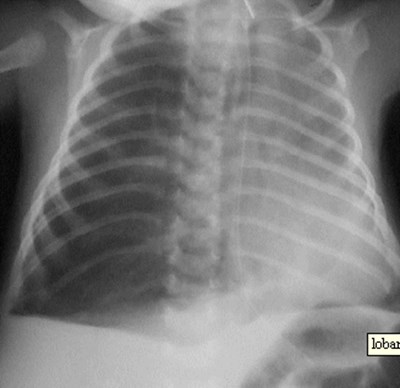
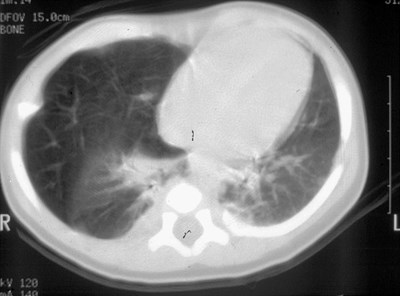
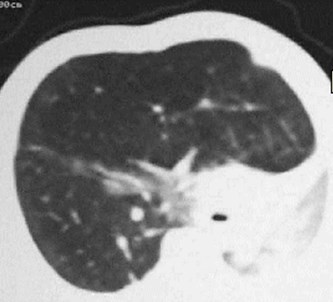
CLE of RML

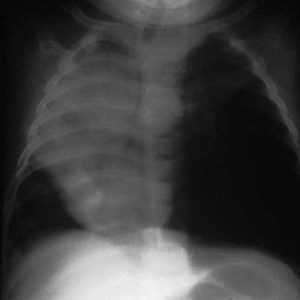
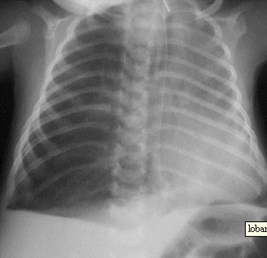
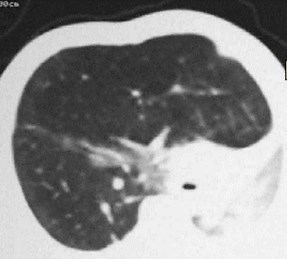
CCAM vs CLE

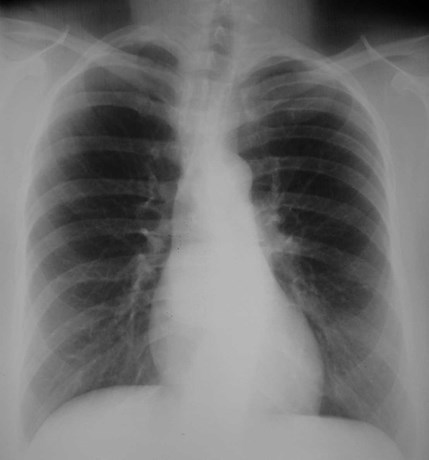

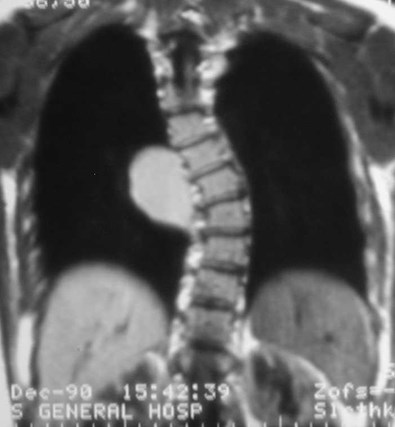
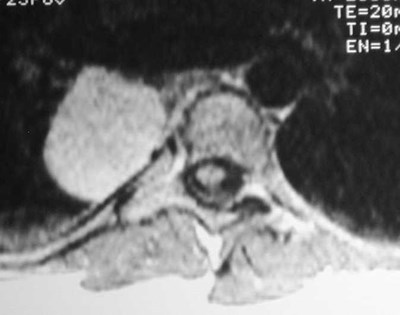
T1WI
T2WI

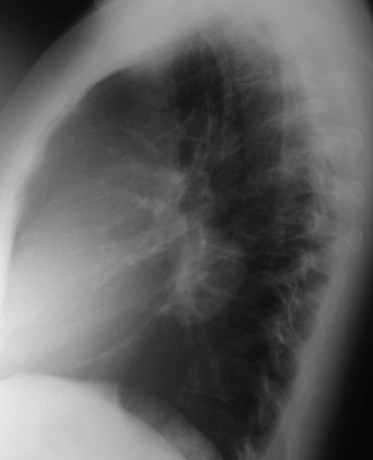
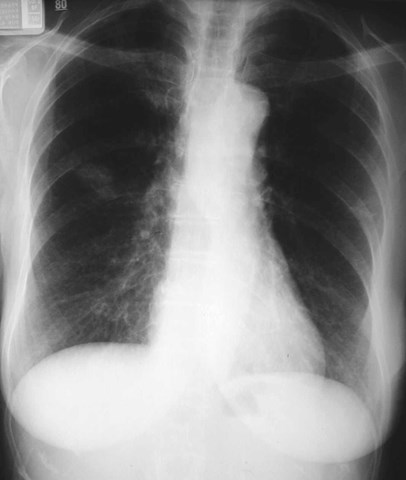
Bronchogenic Cyst
•Mediastinal >> pulmonary (6:1)
•Mediastinal - mostly near carina
•Pulmonary - lower lobes predominate (R=L)
•Usually Asx, may present with infection orcompression effects
•Pulmonary cysts usually contain air
•Cyst contents watery to mucinous, with variableattenuation
•No enhancement!

Bronchogenic Cyst
•Lined by respiratory epithelium
•May have smooth muscle, mucusglands, cartilage in wall
•May have fibrous connection toesophagus or bronchial tree

Bronchogenic Cyst
•May contain a variety of fluids:
–Simple serous fluid (40%)
–Hemorrhagic
–Proteinaceous
–Milk of Calcium
•Histologically often indistinguishablefrom GI duplication cyst

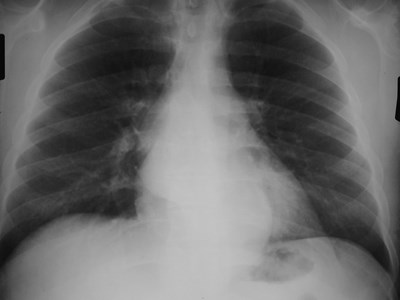
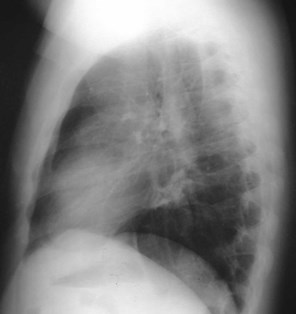
Bronchogenic Cyst - Imaging
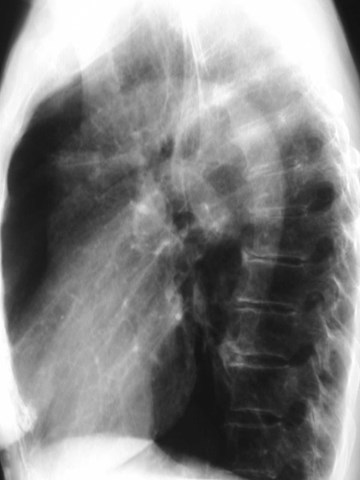
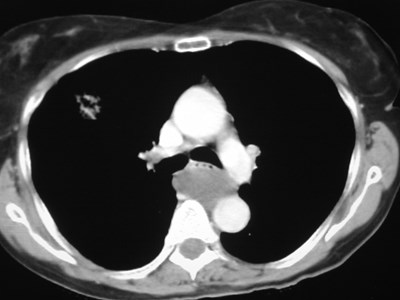
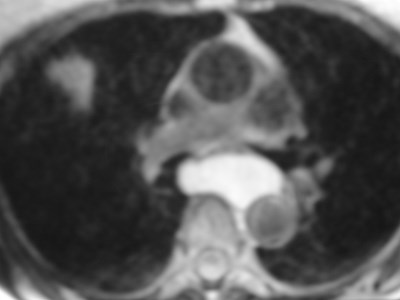
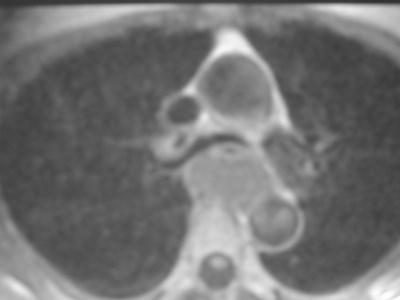
•CXR: retrocardiac/subcarinal oval mass
•CT: smoothly defined, no discernable wall,
–0-60 HU
•MRI: very bright on T2WI, low to high signalon T1WI
•No enhancement with IV contrast


CECT
PDWI
T1WI
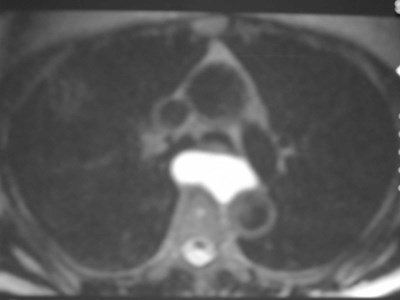
T2WI

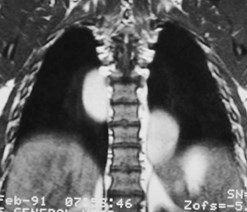
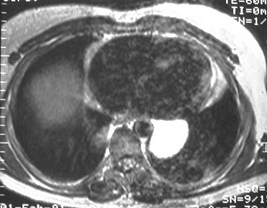
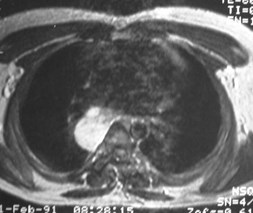

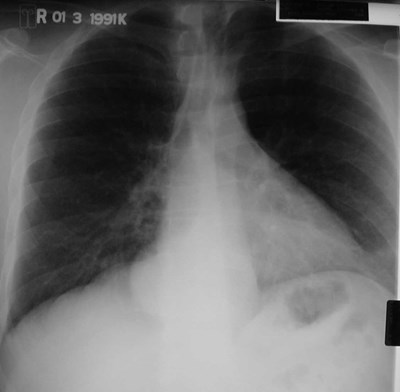
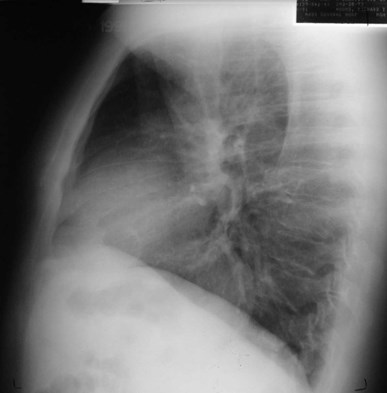
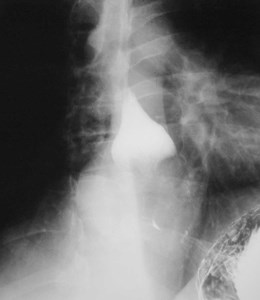
Duplication cyst

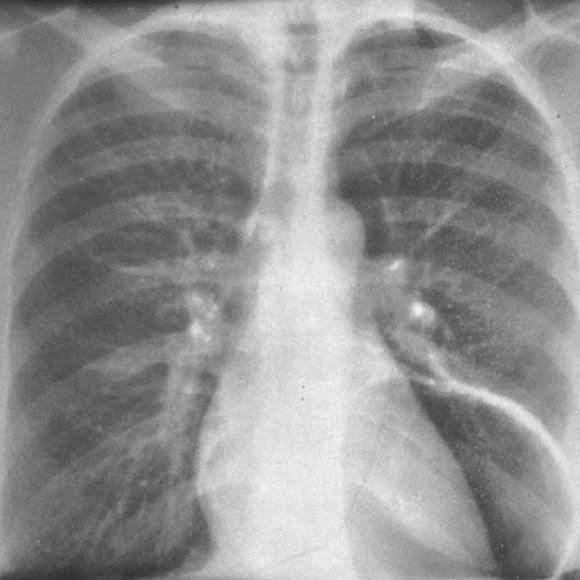




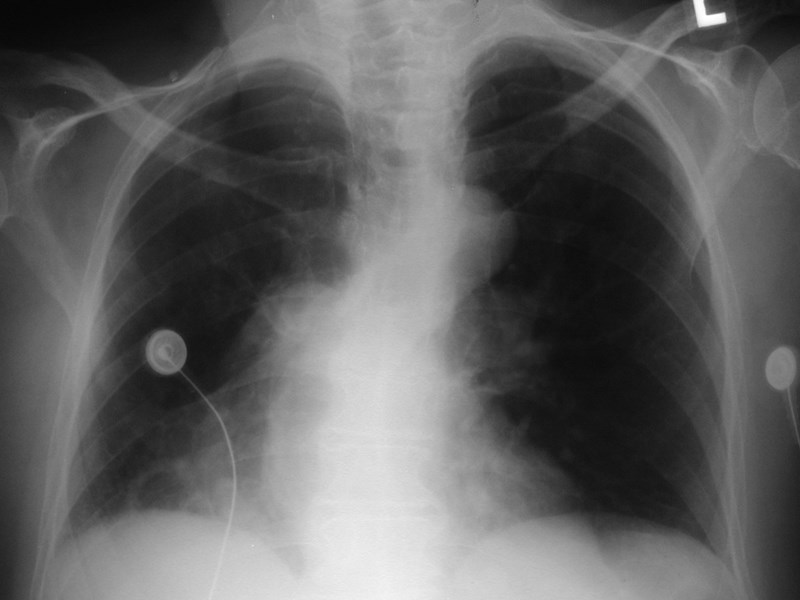
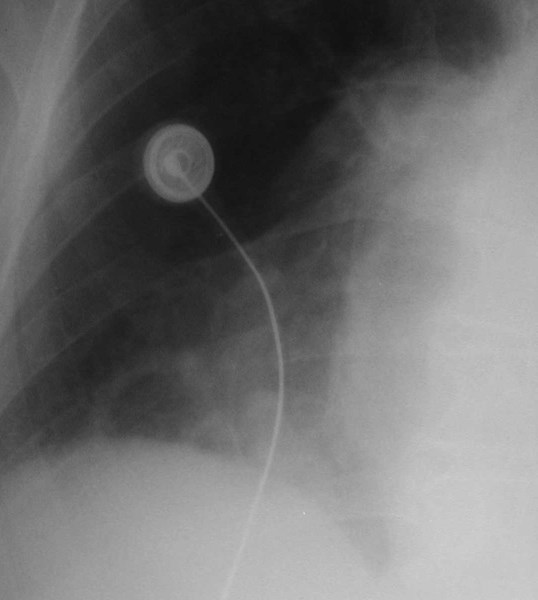




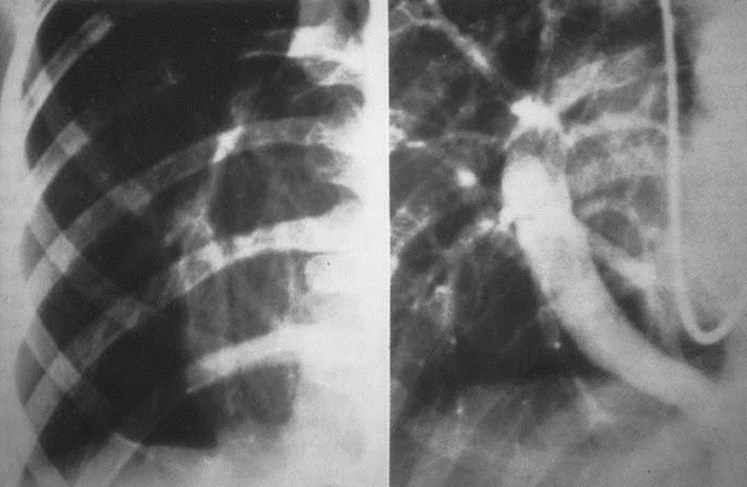

Scimitar Syndrome
•“pulmonary venolobar syndrome”, “hypogeneticlung syndrome”
•Anomalous venous return to IVC
•Systemic arterial supply
–Small RPA
•Abnormal development of right lung, usuallysmall in size
•Abnormal bronchial branching in right lung
•May have abnormal lobation
•Varying degrees of abnormality of parenchyma, arteriesand veins

Scimitar Syndrome
•25% with other C-V anomalies
•1/3 show clear anomalous vein on CXR
•Often Asx, may have:
–Hemoptysis from bronchial collaterals
–Symptomatic L R shunt
–Recurrent infection

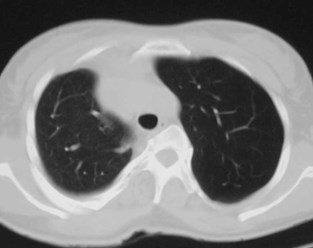
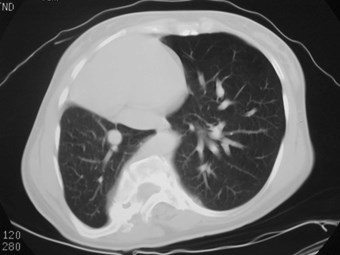
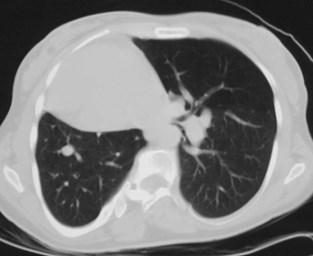
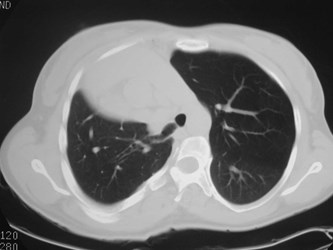

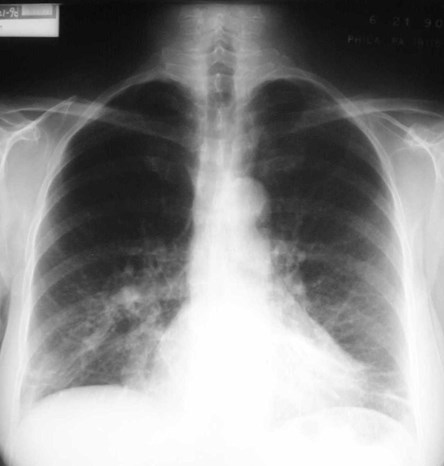
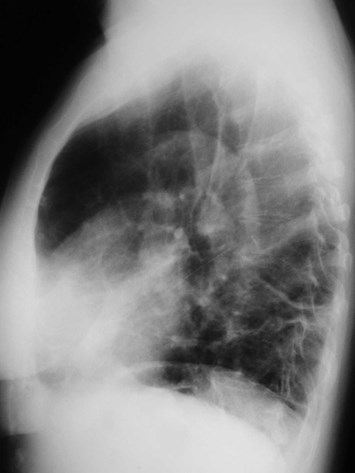

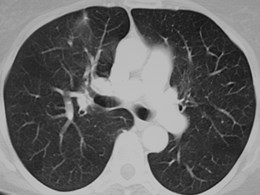
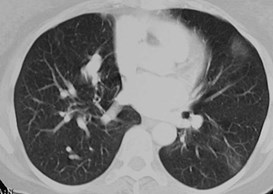
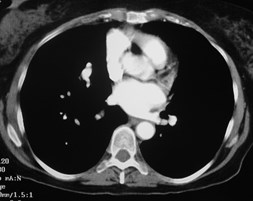
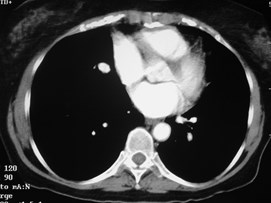
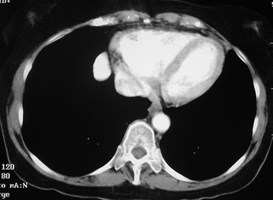
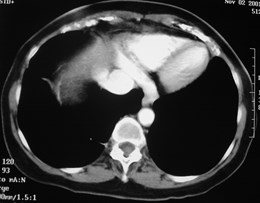

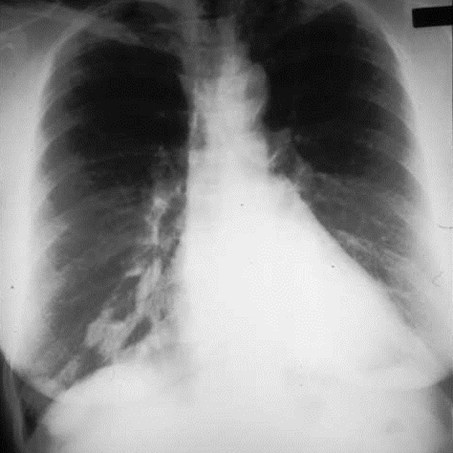
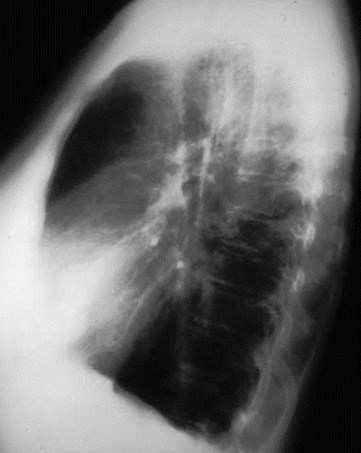

Pulmonary AVM
•Majority congenital
•Persistence of fetal anastamotic capillaries
•Pathology = cavernous hemangioma
•Simple type: single feeding and draining vessels(79%)
•Complex type: multiple feeders, drainers
•~5% with systemic feeders, occasionallysystemic drainage

Pulmonary AVM
•Most found in 3rd or 4th decades
•1/3 found in pts with Osler-Weber-Rendusyndrome
•<20% of O-W-R pts have AVM’s
•1/3 multiple
•8% bilateral

Pulmonary AVM Symptoms & Signs
•50% symptomatic (classic triad: DOE, cyanosis,clubbing)
•R L shunt (murmur or bruit may be heard)
•Paradoxical embolization and brain abscesses
–Loss of lung’s filter function
•Polycythemia due to chronic hypoxia
•Occasional hemoptysis

Pulmonary AVMRadiography
•Plain film: one or more circumscribed nodules.Enlarged feeding and draining vessels may beseen. Ca++ rare
•Grow over time
•CT: CT angiography can now easily showlesions AND establish size and number offeeders
•MRI: GRE or MRA imaging can show flow innodules

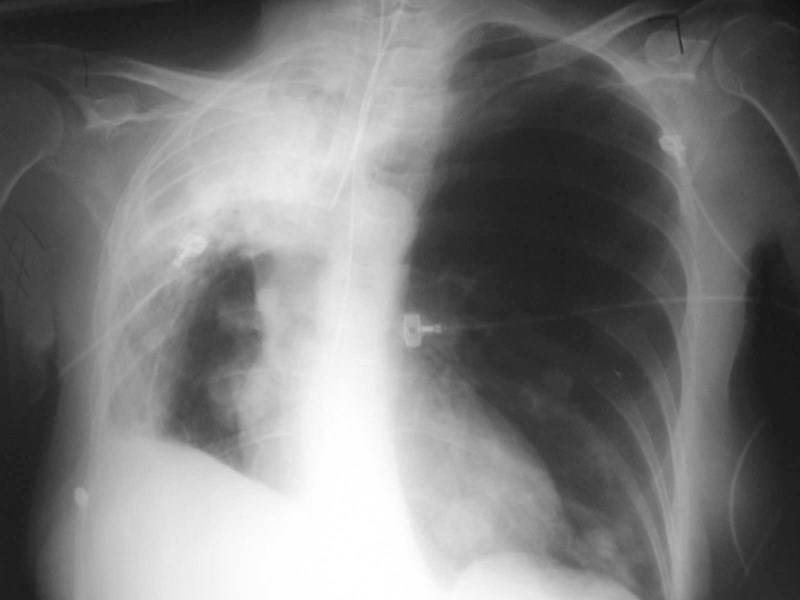

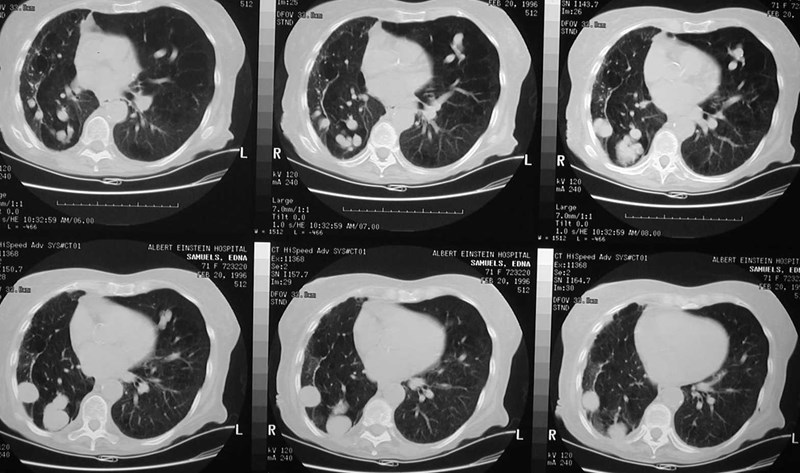

Pulmonary AVM - Treatment
•Ablation recommended for all>3mm
•Angiographic placement of coils,detachable balloons preferred overresection


That’s all, folks!